Draw A Rough Sketch Of The Energy Profile
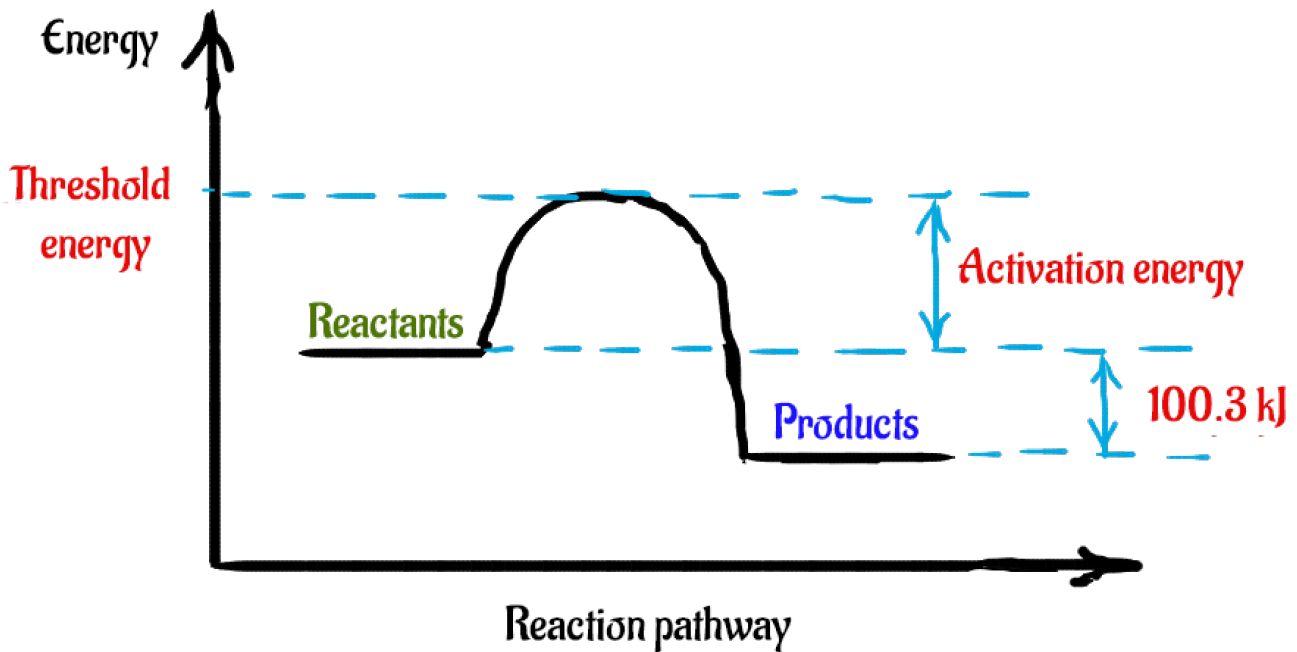
Activation Energy of a reaction E a is the minimum amount of energy reactant molecules must possess in order to form products.
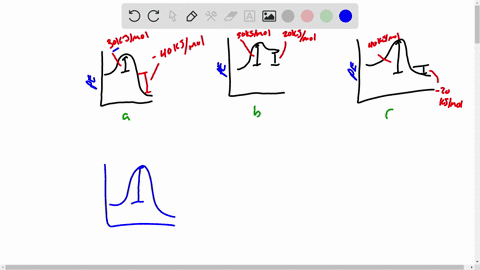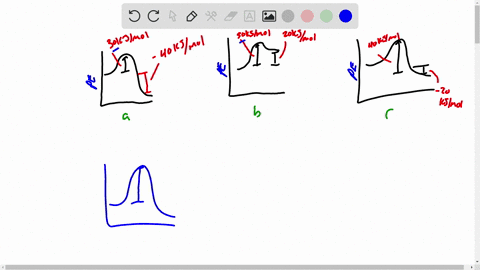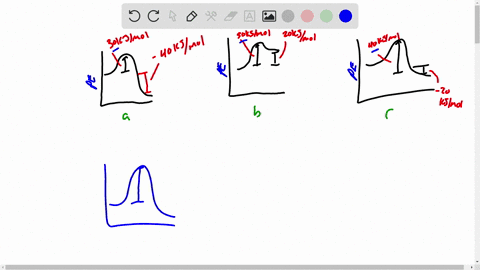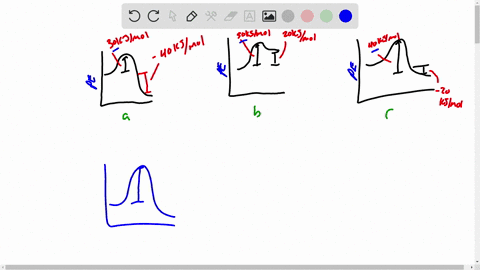
Draw a rough sketch of the energy profile. What scientific concept do you need to know in order to solve this problem. To maintain conservation of energy the potential energy must be. 3 ΔE -50 kJmol E_a50 kJmol.
Draw a rough sketch of the energy profile for each of the following cases. What is the activation energy for the decomposition of HI. On an energy profile the energy of the reactants is lower than the energy of the products.
E -50 KJmol E a 50 kJmol. Draw a rough sketch of the energy profile for each of the following cases. Delta E10 mathrmkJ mathrmmol E_mathrma25 mathrmkJ mathrm.
ΔE -10 kJmol Ea 50 kJmol. The activation energy for the reaction H2g I2g 2HI g is 167 kJmol and E for the reaction is 28 kJmol. Draw a rough sketch of the energy profile for each of the following cases.
E 50 kJmol Ea 50 kJmol. E 10 kJmol Ea 50 kJmolc. Draw a rough sketch of the energy profile for each.
Consider the following energy diagram showing the energy of a reaction as it progresseshow many elementary steps are involved in this reaction. Draw a rough sketch of the energy profile for each of the following cases. See all problems in Energy Diagram.